VISIONS
Critical mass
by Alex Wellerstein, published April 10th, 2015
When we talk about how nuclear weapons work, we inevitably mention the “critical mass.” This is the amount of fissile material you need to create a self-sustaining nuclear reaction. But it’s a very tricky concept, one often poorly deployed and explained, and the result, I have found while teaching and while talking to people online, is an almost universal confusion about what it means on a physical level.
Where does the term come from? In the Smyth Report released in August 1945, the term “critical size” is used almost universally, while “critical mass” is used exactly once (and parenthetically, at that). A more interesting term, the “critical condition,” is used in a few places. The Los Alamos Primer, from 1943, uses critical “radius,” “volume,” “conditions,” in addition to “mass.” The MAUD Report, from 1941, uses critical “size,” “value,” and “amount” — not mass. The Frisch-Peirels memorandum, from 1940, uses critical “radius,” “size,” and “condition.” Leo Szilard’s pre-fission, 1935 patent on chain reacting systems uses the terms critical “thickness,” and “value,” not mass. This is not to imply that people didn’t use the term “critical mass” at the time — but it was one term among many, not the only term. The earliest context I have found it being used extensively comes from a paper in 1941, where it was being used specifically to talk about whether masses of fissile material could be made to explode on demand and not before.
Why use “critical mass” instead of other terms? For one thing, talking about the mass can help you get a sense of the size of the problem when fissile material is scarce and hard to produce (producing fissile material consumed 80% of the Manhattan Project’s budget). And it can also help you when talking about safety questions — about avoiding a nuclear reaction until you absolutely want on. So you don’t want to inadvertently create a critical mass. And knowing that the critical mass is so many kilograms of fissile material, as opposed to so many tons, was an early and important step in deciding that an atomic bomb was feasible in the first place.
What I don’t like about the term, though, is that it can easily lead to confusion. I have seen people assert, for example, that you need a “critical mass” of uranium-235 to start a nuclear reaction. Well, you do — but there is no one critical mass of uranium-235. In other words, used sloppily, people seem to often think that uranium-235 or plutonium have single values for their “critical mass,” and that “a critical mass” of material is what you use to make a bomb. But it’s more complicated than that, and this is where I think focusing on the mass can lead people astray.
Put simply, the amount of fissile material you need to start a nuclear reaction varies by the conditions under which it is being considered. The mass of material matters, but only if you specify the conditions under which it is being kept. Because under different conditions, any given form of fissile material will have different critical masses.
I’ve seen people (mostly online) want to talk about how nuclear weapons work, and they look up what “the” critical mass of uranium-235 is, and they find a number like 50 kg. They then say, OK, you must need 50 kg to start a nuclear reaction. But this is wrong. 50 kg of uranium-235 is the bare sphere critical mass of uranium-235. In other words, if you assembled 50 kg of uranium-235 into a solid sphere, with nothing around it, at normal atmospheric conditions, it will start a self-sustaining chain reaction. It probably would not produce an explosion of great violence — the uranium sphere would probably just blow itself a few feet apart (and irradiate anyone nearby). But once blown apart, the reaction would stop. Not a bomb.
So does that mean that 50 kg of uranium-235 is a important number in and of itself? Only if you are assembling solid spheres of uranium-235.
Is 50 kg the amount you need for a bomb? No. You can get away with much smaller numbers if you change the conditions. So if you put a heavy, neutron-reflecting tamper around the uranium, you can get away with around 10 to 15 kg of uranium-235 for a bomb — a factor of 3-5X less mass than you thought you needed. If your uranium-235 is dissolved in water, it takes very low masses to start a self-sustaining reaction — a dangerous condition if you didn’t mean to start one! And it may be possible, under very carefully-developed conditions, to make a bomb with even smaller masses. (The bare-sphere critical mass of plutonium is around 10 kg, but apparently one can get a pretty good bang out of 3-4 kg of it, if not less, if you know what you are doing.)
Conversely, does this mean that you can’t possibly have 50 kg of uranium (or more) in one place without it detonating? No. If your uranium is fashioned not into a solid sphere, but a cylinder, or is a hollow sphere, or has neutron-absorbing elements (i.e. boron) embedded in it, then you can (if you know what you are doing) exceed that 50 kg number without it reacting. And, of course, there are also impurities — the amount of uranium-238 in your uranium-235 will increase the size of any critical mass calculation.
In other words, under different conditions, the mass of fissile material that will react varies, and varies dramatically. These different conditions include different geometries, densities, temperatures, chemical compositions/phases, and questions about whether it is embedded into other types of materials, whether there are neutron-moderating substances (i.e. water) present, enrichment levels, and so on. It’s not a fixed number, unless you also fix all of your assumptions about the conditions under which it is taken place.
The classic example of this, of course, is the implosion bomb design. The bare sphere critical mass of plutonium-239 is 10 kg. The Nagasaki bomb contained 6.2 kg of plutonium as its fuel. At normal, room-temperature densities, a solid sphere of 6.2 kg of plutonium is not critical. Increase its density by 2.5X through the careful application of high explosives, however, and suddenly that is at least one critical mass of plutonium. Even this is something of an oversimplification, because it’s not just the density that matters: the allotropic (chemical) phase of plutonium, for example, affects its critical mass conditions (and plutonium is notorious for having an unusual number of these phases), and the Nagasaki bomb also included many other useful features meant to help the reaction along like a neutron initiator (which gave it a little shot of about 100 neutrons to start things off), and a heavy, natural-uranium tamper.
What I dislike about the term “critical mass,” as well, is that it can serve to obscure the physical process that defines “criticality.” It can make it seem like reactivity is a function of the mass alone, which is wrong. Worse, it can keep people from realizing why the mass matters in the way it does (among other things). And this can lead to confusion on questions like, “how much explosive power does a critical mass release?” The answer is… it has nothing to do with the critical mass per se. That is a question of bomb efficiency, which can seem like a secondary, separate question. But both the question of criticality and efficiency are really one and the same phenomena — if you understand the underlying physical process on an intuitive level.
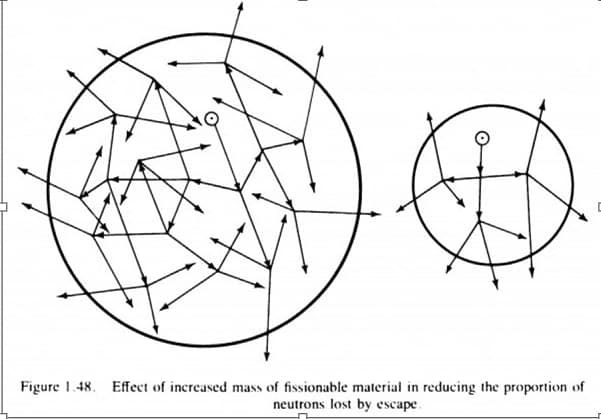
Criticality, the “critical condition,” is defined as the point at which a chain reacting system becomes self-sustaining. So we can imagine a whole sea of uranium-235 atoms. Neutrons enter the system (either from a neutron source, spontaneous fissioning, or the outside world). If they are absorbed by a uranium-235 nucleus, they have a chance of making it undergo fission. That fission reaction will produce a random number (2.5 on average) of secondary neutrons. To be critical, enough of these neutrons will then have to go on to find other uranium nuclei to keep the overall level of neutrons (the “neutron economy”) constant. If that total number of neutrons is very low, then this isn’t very interesting — one neutron being replenished repeatedly isn’t going to do anything interesting. If we’ve already got a lot of neutrons in there, this will generate a lot of energy, which is essentially how a nuclear reactor works once it is up and running.
Supercriticality, which is what is more important for bomb design (and the initial stages of running a reactor) is when your system produces more than one extra neutron in each generation of fissioning. So if our uranium atom splits, produces 2 neutrons, and each of those go on to split more atoms, we’re talking about getting two neutrons for every one we put into the system. This is an exponentially-growing number of neutrons. Since neutrons move very quickly, and each reaction takes place very quickly (on the order of a nanosecond), this becomes a very large number of neutrons very quickly. Such is a bomb: an exponential chain reaction that goes through enough reactions very quickly to release a lot of energy.
So what are the conditions that produce these results? Well, it’s true that if you pure enough fissile material in one place, in the right shape, under the right conditions, it’ll become critical. Which is to say, each neutron that goes into the material will get replaced by at least another neutron. It will be a self-sustaining reaction, which is all that “criticality” means. Each fission reaction produces on average 2.5 more neutrons, but depending on the setup of the system, most or all of those may not find another fissile nuclei to interact with. If, however, the system is set up in a way that means that the replacement rate is more than one neutron — if every neutron that enters or is created ends up creating in turn at least two neutrons — then you have a supercritical system, with an exponentially-increasing number of neutrons. This is what can lead to explosions, as opposed to just generating heat.
In a bomb, you need more than just a critical reaction. You need it to be supercritical, and to stay supercritical long-enough that a lot of energy is released. This is where the concept ofefficiency comes into play. In theory, the Fat Man’s 6.2 kg of plutonium could have released over 100 kilotons worth of energy. In practice, only about a kilogram of it reacted before the explosive power of the reaction separated the plutonium by enough that no more reactions could take place, and “only” released 20 kilotons worth of energy. So it was about 18% efficient. The relative crudity of the Little Boy bomb meant that only about 1% of its fissile material reacted — it was many times less efficient, even though it had roughly 10X more fissile material in it than the Fat Man bomb. The concept of the critical mass, here, really doesn’t illuminate these differences, but an understanding of how the critical reactions work, and how the overall system is set up, does.
This understanding of criticality is more nuanced than a mere mass or radius or volume. So I prefer the alternative phrasing that was also used by weapons designers: “critical assembly” or “critical system.” Because that emphasizes that it’s more than one simple physical property — it’s about how a lot of physical properties, in combination with engineering artifice, come together to produce a specific outcome.
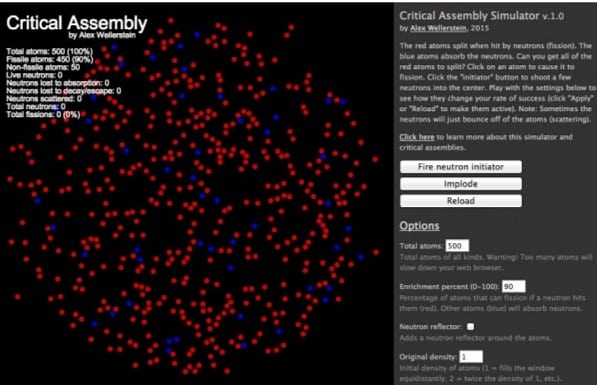
I’ve been playing around with the scripting language Processing.js recently, in my endless quest to make sure my web and visualization skills are up-to-date. Processing.js is a language that makes physics visualizations (among other things) pretty easy. It is basically similar to Javascript, but takes care of the “back end” of graphics to a degree that you can just say, “create an object called an atom at points x and y; render it as a red circle; when it comes into contact with another object called a neutron, make it split and release more neutrons,” and so on. Obviously it is a little more arcane than just that, but if you have experience programming, that is more or less how it works. Anyway, I had the idea earlier this week that it would be pretty easy to make a simple critical assembly “toy” simulation using Processing, and this is what I produced:
The gist of this application is that the red atoms are uranium-235 (or plutonium), and the blue atoms are uranium-238 (or some other neutron-absorbing substance). Clicking on an atom will cause it to fission, and clicking on the “fire neutron initiator” button will inject a number of neutrons into the center of the arrangement. If a neutron hits a red atom, it has a chance to cause it to fission (and a chance to just bounce off), which releases more atoms (and also pushes nearby atoms away). If it hits a blue atom, it has a chance to be absorbed (turning it purple).
The goal, if one can put it that way, is to cause a chain reaction that will fission all of the atoms. As you will see from clicking on it, in its initial condition it is hard to do that. But you can manipulate a whole host of variables using the menu at the right, including adding a neutron reflector, changing the number of atoms and their initial packing density, the maximum number of neutrons released by the fission reaction, and even, if you care to, changing things like the lifetimes of the neutrons, the likelihood of the neutrons just scattering off of atoms, and whether the atoms will spontaneously fission or not. If you have a reflector added, you can also click the “Implode” button to make it compress the atoms into a higher density.
This is not a real physical simulation of a bomb, obviously. None of the numbers used have any physically-realistic quality to them, and real atomic bombs rely on the fissioning of trillionsof atoms in a 3D space (whereas if you try to increase the number of atoms visible to 1,000, much less 10,000, your browser will probably slow to a crawl, and this is just in 2D space!). And this simulator does not take into account the effects of fission products, among other things. But I like that it emphasizes that it’s not just the number of atoms that determines whether the system is critical — it’s not just the mass. It’s all of the other things in the system as well. Some of them are physical constants, things pertaining to the nature of the atoms themselves. (Many of these were constants not fully known or understood until well after 1939, which is why many scientists were skeptical that nuclear weapons were possible to build, even in theory.) Some of them are engineering tricks, like the reflector and implosion.
My hope is that this kind of visualization will help my students (and others) think through the actual reaction itself a bit more, to help build an intuitive understanding of what is going on, as a remedy to the aspects of a prior language that was created by scientists, diffused publicly, and then got somewhat confused. “Critical mass” isn’t a terrible term. It has its applications. But when it can lead to easy misunderstandings, the language we choose to use matters.

Gordon Duff posted articles on VT from 2008 to 2022. He is a Marine combat veteran of the Vietnam War. A disabled veteran, he worked on veterans and POW issues for decades.
Gordon is an accredited diplomat and is generally accepted as one of the top global intelligence specialists. He manages the world’s largest private intelligence organization and regularly consults with governments challenged by security issues.
Duff has traveled extensively, is published around the world, and is a regular guest on TV and radio in more than “several” countries. He is also a trained chef, wine enthusiast, avid motorcyclist, and gunsmith specializing in historical weapons and restoration. Business experience and interests are in energy and defense technology.
ATTENTION READERS
We See The World From All Sides and Want YOU To Be Fully InformedIn fact, intentional disinformation is a disgraceful scourge in media today. So to assuage any possible errant incorrect information posted herein, we strongly encourage you to seek corroboration from other non-VT sources before forming an educated opinion.
About VT - Policies & Disclosures - Comment Policy